

Kinetic vs. Thermodynamic Control in Organic Reactions
The concept of kinetic vs. thermodynamic control of reactions is an important one in organic chemistry. There are a number of reactions known in which there are two (or more) possible reaction products of a reaction, and one product (kinetic product) predominates when the reaction is done at low temperature. The other (thermodynamic product) predominates when the reaction is done at a higher temperature. This concept is investigated using the CACheTM molecular modeling software.
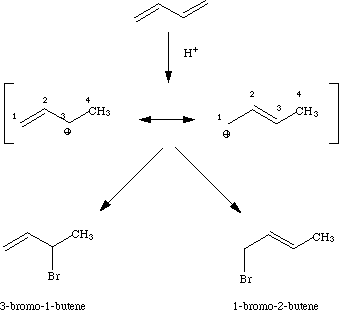
I. Addition of HBr to 1,3-Butadiene
HBr adds to 1,3-butadiene to form a mixture of 3-bromo-1-butene (1,2-addition) and 1-bromo-2-butene (1,4-addition).1 At -80 oC, 3-bromo-1-butene is the major product, and at 40 oC 1-bromo-2-butene is the major product.
Procedure
In the CAChe Editor, draw the structures of the allylic carbocation (either resonance form), 3-bromo- 1-butene and 1-bromo-2-butene (cis and trans isomers). Be certain to have the correct hybridization for the carbon atoms and a +1 charge on the carbocation. You need not draw hydrogen atoms. From the Beautify menu select Comprehensive to obtain a drawing of the molecule with hydrogens added and approximately correct bond angles.
Minimizing the energy of structures is done by selecting Applications and then MOPAC. Select Optimize Geometry under calculation type and AM1 parameters. Run the program and obtain the value of the heat of formation found in the dialog box.
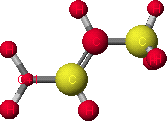
Representation of allylic carbocation color coded to show partial charges
In order to view the charge density of the carbocation, go to Applications and select Visualizer. From the View menu select Atom Shape. Set the parameters in the dialog box to display Element and Charge as Spheres, shaded and radius for partial charge. The partial charge on each atom will be displayed color-coded with red representing partial positive charge and yellow representing partial negative charge. The size of sphere is proportional to the amount of partial charge on the atom. Alternatively numerical values of the partial charge on each atom and heats of formation for all species can be obtained using Project LeaderTM.Results
Results of these calculations show that in the allylic carbocation intermediate there is more partial positive charge on C-3 (+0.29) than on C-1 (+0.12). Thus under kinetic conditions nucleophilic attack by bromide occurs at the secondary allylic carbon rather than the primary allylic carbon. The calculated heats of formation verify that 1-bromo-2-butene (thermodynamic product) is more stable than 3-bromo-1-butene.
| Compound | Heat of Formation (kcal/mole) |
|---|---|
| 3-bromo-1-butene | 6.40 |
| trans-1-bromo-2-butene | 0.89 |
| cis-1-bromo-2-butene | 1.95 |
II. Sulfonation of Naphthalene
Another reaction that illustrates the concept of kinetic vs. thermodynamic ontrol is the sulfonation of naphthalene with SO3 in H2SO4.2 When the reaction is run at 80 oC, 1-naphthalenesulfonic acid is the major product; at 160 oC, 2-naphthalenesulfonic acid is the major product. The intermediates in the sulfonation are arenium ions. The relative energies of the arenium ions should approximate the energies of the transition states leading to the formation of the sulfonic acids (Hammond Postulate). 2-Naphthalenesulfonic acid is more stable than 1-naphthalenesulfonic acid because of an unfavorable steric interaction between the sulfonic acid group in the 1-position and the hydrogen in the 8-position. The arenium ion leading to 1-naphthalenesulfonic acid is more stable than the arenium ion leading to the 2-isomer because of better resonance stabilization.
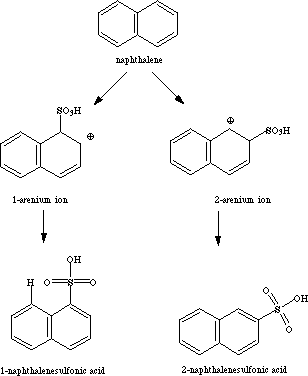
The reaction should be able to be modeled by calculating the heats of formation of the isomeric naphthalenesulfonic acids and the arenium ion intermediates leading to each.3
Procedure
In the CAChe Editor, construct structures for 1-naphthalenesulfonic acid, 2-naphthalenesulfonic and the 1-arenium ion and 2-arenium ions shown above. Then use MOPAC to minimize the energy of each and calculate the heat of formation of each.
Results
Calculation of the heats of formations gave results that are in agreement with expectation, as shown in the table below.
| Structure | Heat of Formation (kcal/mole) |
|---|---|
| 1-Naphthalenesulfonic acid | -75.36 |
| 2-Naphthalenesulfonic acid | -77.32 |
| 1-Arenium ion (precursor to 1-naphthalenesulfonic acid) | 111.68 |
| 2-Arenium ion (precursor to 2-naphthalenesulfonic acid) | 112.75 |
References
- L.G. Wade, Organic Chemistry (Prentice-Hall, 1987) 569-72.
- P.Y. Bruice, Organic Chemistry (Prentice-Hall, 1995) 619-20.
- W.J. Hehre, A.J. Shusterman, W.W. Huang, A Laboratory Book of Computational Organic Chemistry (Wavefunction, 1996), 177.