

Dehydration of 2-Methylcyclohexanol
Brian W. Moores
Background
The acid-catalyzed dehydration of 2-methylcyclohexanol to form a mixture of cyclic alkenes has become a standard experiment in the organic chemistry and upper-level laboratory curricula at several colleges and universities (1-4). Among other things, it illustrates the utility of Zaitsev's rule. The mechanism is most likely that shown at the right.
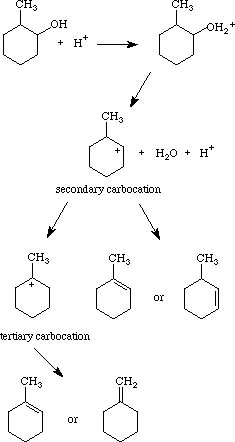
The distribution of products seems to depend on (a) the relative rates of reaction of the cis and trans isomers of the starting alcohol, (b) the relative stabilities of the secondary and tertiary carbocations, and (c) the length of time over which the reaction is carried out. These phenomena reflect the relative influences of thermodynamic vs. kinetic control over the progress of the reaction. Kinetically, the reaction of the alcohol cis isomer has a rate constant at least eight times that of the trans isomer (3). Thermodynamically, the formation of 1-methylcyclohexene is preferred because of its lower enthalpy of formation; the next most exothermic enthalpy of formation is that of 3-methylcyclohexene, and the least negative enthalpy of formation is that of methylenecyclohexane. Furthermore, the greater stability of the tertiary carbocation over the secondary cation makes the final step of the reaction thermodynamically somewhat reversible, so that if the reaction is carried out over longer times, a relatively larger fraction of methylenecyclohexane (compared to 1-methylcyclohexene) is produced, even though methylenecyclohexane has the least exothermic enthalpy of formation of the products.
Molecular modeling is a tool that can allow students to predict the relative thermodynamic stabilities of the intermediates and products of this reaction, and also to produce three-dimensional structures which show the lowest-energy geometries of these intermediates and products.
This experiment therefore offers a useful illustration of the following concepts and experimental techniques:
- dehydration of a secondary alcohol;
- use of gas chromatography to separate and quantify a mixture of products;
- use of mass-spectrometric detection as a tool to identify the compounds giving rise to gas-chromatographic peaks;
- concept of kinetic vs. thermodynamic control;
- use of modern computational techniques to predict experimental results.
Experimental
The experimental details for carrying out the dehydration are given in several references (1, 3, 4).
Molecular Modeling
The organic molecules or molecule-ions involved in this reaction were constructed using both software applications CACheTM and MacSpartanTM, and their geometries optimized using semiempirical molecular orbital calculations. When CAChe was used, the PM3 calculation was used to optimize geometry, while AM1 was used as the optimization routine on MacSpartan. The following results were obtained:
| Compound | PM3 Enthalpy of Formation (kcal/mol) | AM1 Enthalpy of Formation (kcal/mol) |
|---|---|---|
| 2-methylcyclohexanol | -75.783 | -88.559 |
| 1-methylcyclohexene | -14.426 | -17.832 |
| 3-methylcyclohexene | -10.293 | -14.846 |
| methylenecyclohexane | -9.809 | -12.686 |
| tertiary carbocation | 168.28 | 158.06 |
| secondary carbocation | 181.02 | 169.04 |
Both semiempirical methods predict the expected order of enthalpies of formation of the three hydrocarbon products, and also predict the expected order of thermodynamic stability of the carbocation intermediates.
Students can see that the geometry about the carbon atom carrying the positive charge is trigonal planar, as expected from sp2 hybridization of the carbon atom.
References
- Taber, Richard L.; Champion, William C., J. Chem Educ, 1967, 44(10), 620.
- Todd, David, J. Chem Educ, 1994, 71(5), 440.
- Cawley, John J.; Lindner, Patrick E., J. Chem Educ, 1997, 74(1), 102.
- Poon, Thomas; Mundy, Bradford P.; McIntyre, Jean; Woods, Lesley, J. Chem Educ, accepted for publication.