

An Animated View of the Polymerization of Sulfur
Introduction
The polymerization of sulfur (aka plastic sulfur) has long been used as a demonstration in chemistry classes. I use it with freshman to try to link macroscopic observations with microscopic explanations. I use it again with physical chemistry students as an example of a substance with an interesting phase diagram and as an illustration of the conversion of substances into their most thermodynamically stable form in order to minimize chemical potentials. I have developed an animation of the demonstration with CACHE and GifBuilder to be used in conjunction with the demonstration in order to help students visualize the microscopic behavior that occurs during the heating of the sulfur.
The Demonstration
The supplies and equipment needed for the experiment are:
- Powdered sulfur [Precipitated sulfur works well. Grinding solid sulfur in a mortar and pestle works best but charges during crushing (so is annoying at times). Avoid sublimated sulfur.]
- Fisher burner with striker
- Large test tube and appropriate holder
- 500 mL beaker with 300-400 mL of water
- Damp paper towel (to quench any sulfur flames and reduce SO2 production)
Put about 2 inches of sulfur in the test tube and heat slowly over the burner. Once melted, tip tube to show students the viscosity of the melted sulfur. Continue to heat. Typically, as liquids heat, the viscosity drops. Periodically stop and show the viscosity of the sulfur while continuing to heat. At some point, the viscosity changes markedly and the sulfur becomes like molasses. Show this viscosity. As the heating is reapplied, the viscosity will once again drop right before boiling. This change in viscosity is reversible and can be seen by letting the tube cool and the molasses reappear and disappear. Reheat the tube above the viscous state and then pour into the beaker of water. The sulfur will solidify in streams of rubbery material. Allow the students to observe (ie feel) the plastic sulfur. Keep a portion of the plastic sulfur for the next class. It will revert back to the yellow, rhombic form of sulfur (but not in crystalline form) and be hard again.
The Animations
The first animation shows the shape of the elemental sulfur, S8 rings. It rotates to show students both the ring structure and the crown shape it resembles when viewed on its side.
Click on the graphic to see the first animation.
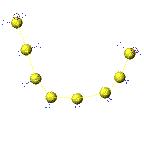
The second animation shows what happens to the ring as heat is applied to the melted sulfur. The increase in temperature is accompanied by an increase in motion and a bond within the ring become strained and finally broken. As the covalent bond breaks equally in half, a diradical is formed.
Click on the graphic to see the second animation.

The third animation shows the coming together of two radicals to form a long chain. This is the polymerization process. It is easier for the molecule to find radicals on other molecules than to find the radical on the other end of its own chain. Polymerization occurs to form long chains.
Click on the graphic to see the third animation.
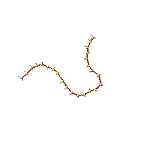
The fourth animation shows the presence of many polymeric chains and the effect on the motion of the sulfur chain. This illustrates the increase in viscosity observed when the polymers are formed. The last frame shows the presence of many chains in the sample.
The fifth animation zooms in on a region of the tangled mess and illustrates what happens when the polymer continues to be heated. The rise in kinetic energy once again causes bonds to be stretched, weakened and broken, resulting in a liquid that contains small fragments of sulfur chains. The viscosity is easily shown to be lower at this point than with the polymeric sulfur by the ease of its motion. When sulfur boils, it has been observed to contain not only S8 but smaller fragments such as S4 as well.