

Equilibrium Constant for the Dimerization of Pinacyanol Iodide
R. Viswanathan
An aqueous solution of pinacyanol iodide can be analyzed using visible spectroscopy to determine the equilibrium constant for the dimerization reaction:
2(pinacyanol) ---> (pinacyanol)2
The experimental wavelength of maximum absorption for the monomer is around 600 nm, while that for the dimer is around 400 nm. The absorption maximum at 600 nm is used to determine the concentration of monomer in the aqueous solution by making use of Beer's law. The dimer concentration is calculated using the equilibrium equation, the monomer concentration, and the initial total concentration. The peak at 400 nm is not used to determine the dimer concentration since the monomer has a significant absorption at this wavelength also. The equilibrium constant is then given by:
This can be combined with a molecular modeling module to determine the electronic absorption spectra of these molecules. This would especially be useful since it is experimentally difficult to obtain the individual absorption spectra. The monomer and dimer cations have been modeled using Cache modeling software. The structures were optimized first using molecular mechanics and then using the semi-empirical AM1 method. The minimized structures are shown below.
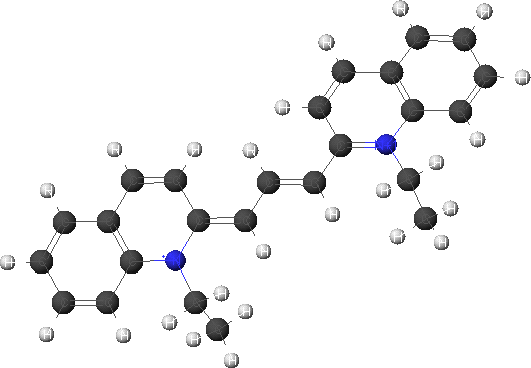
Monomer
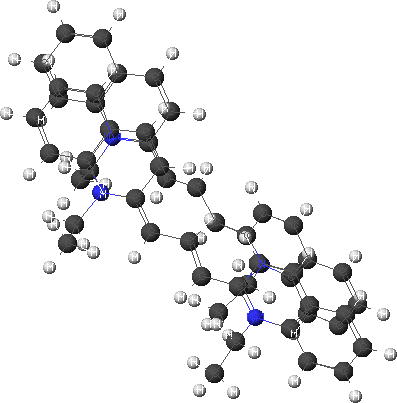
Dimer
The electronic absorption spectra of these two molecules were calculated using ZINDO and the absorption spectra are shown below:
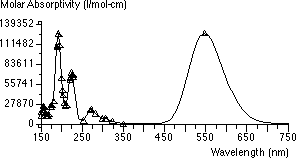
Monomer Absorption Spectrum
The monomer absorption spectrum is closer to the experimental results than the dimer. However, it is clearly seen that the dimer absorbance has shifted towards shorter wavelengths, and the contribution of the dimer to the absorbance at a wavelength of 600 nm, where the monomer absorbs strongly, is minimal. Hence 600 nm can be used for a quantitative determination of the monomer. This modeling exercise clearly indicates why a wavelength of 600 nm should be chosen for quantitative measurements in this exercise.