

Spectra of Dyes and the Particle-in-the-Box
George Nieman
Purpose: In this experiment we will investigate the UV-Vis (electronic) spectra of several dye molecules. In addition to examining the spectra we will model this system by two methods: the particle in a box and molecular modeling in the program CAChe.
Experiment: The dyes to be used in this experiment are:
3,3'-diethylthiacyanine, 3,3'-diethylthiacarbocyanine, 3,3'-diethylthiadicarbocyanine, and 3,3'-diethylthiatricarbocyanine. These dyes are available commercially from Aldrich Chemical Company.
Draw structures for each of these molecules. You may wish to consult the Aldrich catalog for this. The chromophore in these molecules is a conjugated chain of alternating single and double bonds between the two nitrogen atoms.
A sample structure is represented below.
Each student will work with a different dye and then the class will compare their results. Prepare 10.0 mL of solution which is approximately 0.001 M in dye using spectroscopic grade methanol as the solvent. Do this with volumetric glassware and record the actual concentration. Record the UV-Vis spectrum of this solution. If the absorbance exceeds 1.0 (which it will) you will need to dilute this solution and record another spectrum. Make quantitative dilutions by factors of ten and take the spectrum until the maximum absorbance is around 1.0. Be sure to keep track of these dilutions. You will need to know the wavelength of maximum absorbance and the absorptivity at this wavelength. (Note: A=abc)
Theory (box): A very simple model for this system is the "particle in a box." Light absorption occurs when an electron moves from the highest filled to the lowest unfilled orbital. We will represent the conjugated system in these dyes as electrons in a one-dimensional box. The energy levels for this system are given by:
En = n2h2/8mL2,
where n is the orbital number, h is Planck's constant, m is the electron mass, and L is the length of the box.
Since the spectrum represents a transition from one energy level to another you will need to calculate the energy of two adjacent levels and convert their differences into a wavelength (l).
DE = hc/l = En+1 - En.
Electrons go into orbitals two at a time, so the next problem is to calculate the number of r electrons. Each carbon in the chain contributes one r electron. The nitrogen atoms contribute one or two electron depending upon whether they are positive or neutral. (Note that you have one of each.)
There are several ways to estimate the length (L) of the box. One is to assume 120 degree angles at the carbon atoms and tabulated single and double bond lengths. Using geometry it is possible to calculate the direct distance between the two nitrogens. You will need to add to this distance the radius of the nitrogen atom. You should also make a second estimate of L by assuming that the electron travels along the backbone.
Calculate the length of the box representing your dye molecule by the two models suggested, the number of r electrons, the orbitals involved in the transition, and finally the wavelength of the expected transition. Be very careful of the units as you do these calculations. You may wish to set up an Excel spreadsheet to help with these calculations. These calculations will allow you to predict the lmax, but not the molar absorptivity.
Theory (modeling): As a second method to represent the spectra of these dyes we will use the chemical modeling program CAChe. Open the CAChe Editor and construct a model molecule to represent your dye. You should use a model compound rather than the full molecule to lower the calculation times and to avoid limitations in the calculation engines. In particular you want to leave off the aromatic rings. Note that one of the nitrogens has a positive charge and the other is neutral.
A sample model compound is represented below.
Once you have constructed the model compound save it. Also measure various bond lengths (C to C, C to N, and N to N) for use later. Then use the Mechanics application to minimize the geometry. Next open Project Leader and open the structure file(s) you want to work with. Calculate the UV-vis spectrum using the standard method. (You may wish first to optimize the geometry again.) In order to see the spectra you have predicted run the Visualizer application and open the appropriate molecule files. A sample of this output is give below.
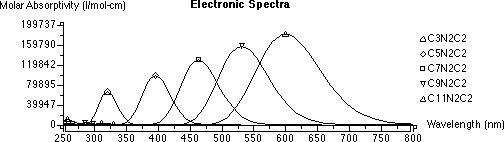
You can return to the Editor application and again measure various bond distances: C to C and N to N. How do these distances compare with you original estimates? Repeat your particle in a box calculations using these revised distances.
Report: Compare both the lmax and absorptivity from the experiment and your model calculations from CAChe. Also compare the calculated lmax for the particle in a box model using various lengths (direct N-to-N, backbone, and CAChe distances.) Gather similar data from the others in the lab and comment on the accuracy of the various models you have used. Are the absolute numbers good? Are the trends represented well?
It is possible to include the sulfur atoms in the model compounds. The spectra
predicted by CAChe for these model compounds are given below.