

Sunscreen Analysis Using Molecular Modeling
Carl Wigal
Reference
We have published an experiment which correlates spectrophotometric analysis of sunscreens with their SPF values:
Walters, C.; Keeney, A.; Wigal, C.; Johnston, C; R. Cornelius, R. "The Spectrophotometric Analysis and Modeling of Sunscreens," J. Chem. Educ., 1997, 74, 99-102.
Introduction
Active ingredients is a term familiar to everyone. The structures for the active ingredients in sunscreens are shown in Figure 1. What properties do these compounds have that make them active ingredients? In this exercise, we will use molecular modeling to examine the relationship between the structure of compounds and their spectral properties. The following pages contain seven questions for you to answer in your notebook.
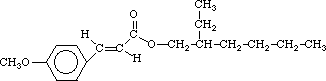
2-ethylhexyl-p methoxycinnamate
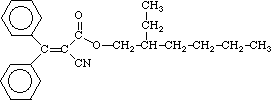
octocrylene
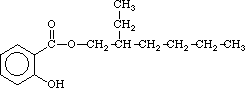
2-ethylhexylsalicylate
oxybenzone
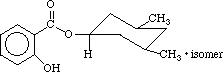
homosalate
Figure 1. Structures of the active ingredients in sunscreens
Modeling
Molecular modeling is a tool used by chemists to predict the structure and properties of compounds based on mathematical models. One such property that can be predicted is the electronic, or ultraviolet-visible (UV-Vis) spectrum of a compound. As an example of a compound for which we can predict the UV-Vis spectrum, we use lycopene, the red pigment in tomatoes. The structure of lycopene and the predicted electronic spectrum based on modeling with CAChe software are shown below. Experimentally, the UV-Vis spectrum of lycopene shows the wavelength of maximum absorbance (lmax) occurs at 476 nm with a molar absorptivity of 191,000 M- 1 cm- 1.
Using CAChe
Use Figure 2 and the known experimental result to answer the first question: 1. How close are the modeling predictions to the actual values?
Figure 2. Structure and predicted electronic spectrum of lycopene
Although modeling gives a fairly accurate prediction of the spectra, the numbers are not absolute. As discussed in your laboratory handout, the UVB region (280-315 nm) of the electromagnetic spectrum is responsible for sunburn. For a compound to act as a sunscreen, it should have an absorbance of high molar absorptivity in this spectral range.
Launch the CAChe Editor from the Apple Menu. Select Visualizer from the Applications menu. Open the structure for 2-ethylhexyl p-methoxycinnamate by selecting the filename p-methoxycinnamate from the SUNSCREEN FOLDER found on the hard disk and click OK. The structure for the sunscreen should now be visible. Select Electronic Spectra from the Analyze menu. The predicted electronic spectrum should now be visible. Maximize the window size of the electronic spectrum by clicking the top right box of the window. Double click on the x-axis of the spectrum. Set the parameters as shown in Figure 3 and click OK.
Figure 3. Settings of axis parameters for 2-ethylhexyl p-methoxycinnamate
We have now defined the absorbance window from 250 to 350 nm which gives us the UVB region of the spectrum plus some room for error. From the predicted spectrum answer the next question: 2. What is the predicted wavelength for the maximum absorbance of 2-ethylhexyl p-methoxycinnamate?
Many sunscreen formulations contain more than one active ingredient. For example, Coppertone SPF 8 contains both 2-ethylhexyl p-methoxycinnamate and oxybenzone. Select Open from the File menu. Open the file oxybenzone from the sunscreen folder. The structure for oxybenzone should now be visible. Select Electronic Spectra from the Analyze menu. The predicted electronic spectra for both compounds should now be visible. 3. What advantage does the combination of two different active ingredients offer over a single ingredient?
Coppertone SPF 45 contains two more active ingredients in addition to those found in SPF 8. These additional compounds are octocrylene and 2-ethylhexyl salicylate. Open the structure and electronic spectrum for each of these compounds as described above. The predicted electronic spectrum for all four compounds should now be visible. 4. Why is this formulation more effective than the formulation of SPF 8?
Select Close from the File menu. Repeat this process until no molecules or spectra are visible.
Examine the structures for the five active ingredients in sunscreens. What do they have in common? As you can see, all compounds contain both an aromatic ring and a carbon-oxygen double bond. The parent compound containing the aromatic ring is called benzene, which is shown below.
benzene |
benzoic acid |
PABA |
| Figure 4. Structures of benzene and related compounds | ||
In Visualizer, open the structure of benzene by selecting Open from the File menu. Select Electronic Spectra from the Analyze menu. Double click on the x-axis of the spectrum. Set the parameters as shown in Figure 5 and click OK.
5. Would benzene be an effective sunscreen? Explain why or why not.
Another compound which contains both an aromatic ring and a carbon-oxygen double bond is benzoic acid. Open the structure and electronic spectrum for benzoic acid. 6. Would benzoic acid be an effective sunscreen? Explain why or why not.
Figure 5. Settings of axis parameters for benzene
In the past, p-aminobenzoic acid (PABA) was used in sunscreen formulations. Open the structure and electronic spectrum for PABA. 7. How does substituting the benzene ring with an amino group (-NH2) affect the spectrum?
As you can see, molecular modeling can be an effective tool in predicting properties of molecules. Select Quit from the File menu when you have finished.